15 of 16
Back to themesLaboratories for New Skills
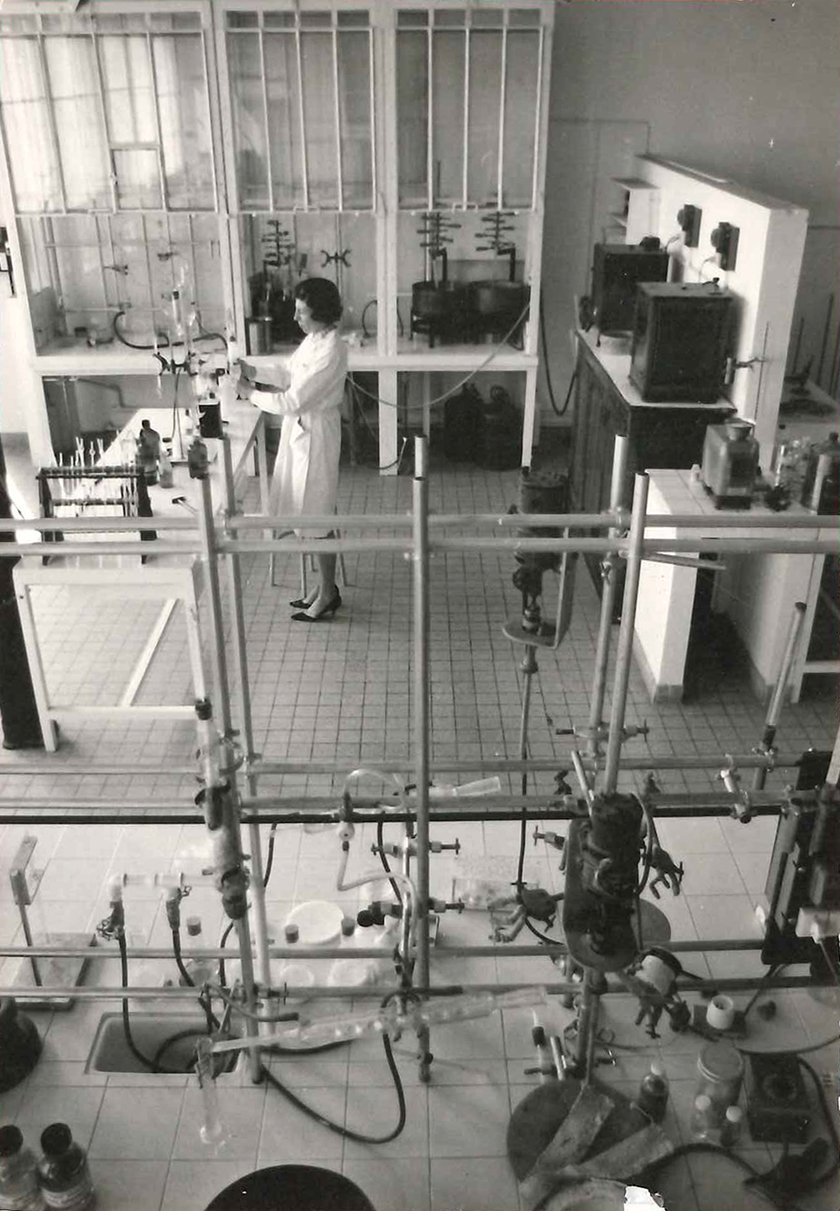
1973 • 2012Gattefossé has always looked to acquire the research equipment it needs to fulfill its ambitions. At the start of the 20th century, the research laboratory was just a lean-to in the garden of the family home. In the 1920s, the factory boasted an expansive space for research and formulation activities. In 1973, Gattefossé opened a “pilots and processes” facility, and in 2012, a cell culture laboratory.